-
It uses the recombinant form of the nucleocapsid protein as the antigen to bind any antibodies in the sample. Which are then detected in scaled up version of an ELISA.
The data is based on 215 women in a hospital in NY.
I think.
https://www.nejm.org/doi/full/10.1056/NEJMc2009316All the women were screened on admission for symptoms of Covid-19. Four women (1.9%) had fever or other symptoms of Covid-19 on admission, and all 4 women tested positive for SARS-CoV-2 (Figure 1). Of the 211 women without symptoms, all were afebrile on admission. Nasopharyngeal swabs were obtained from 210 of the 211 women (99.5%) who did not have symptoms of Covid-19; of these women, 29 (13.7%) were positive for SARS-CoV-2. Thus, 29 of the 33 patients who were positive for SARS-CoV-2 at admission (87.9%) had no symptoms of Covid-19 at presentation.
Edit
Correctionthat paper shows the importance.
This is the test data
https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html#productInfoClinical specificity28
A total of 5,272 samples (from diagnostic routine, blood donors, a common cold panel, and a coronavirus panel*) obtained before December 2019 were tested with the Elecsys® Anti-SARS-CoV-2 assay.
Clinical sensitivity28
A total of 204 samples from 69 symptomatic patients with a PCR confirmed SARS-CoV-2 infection were tested with the Elecsys® Anti-SARS-CoV-2 assay. One or more consecutive specimens from these patients were collected after PCR confirmation at various time points.
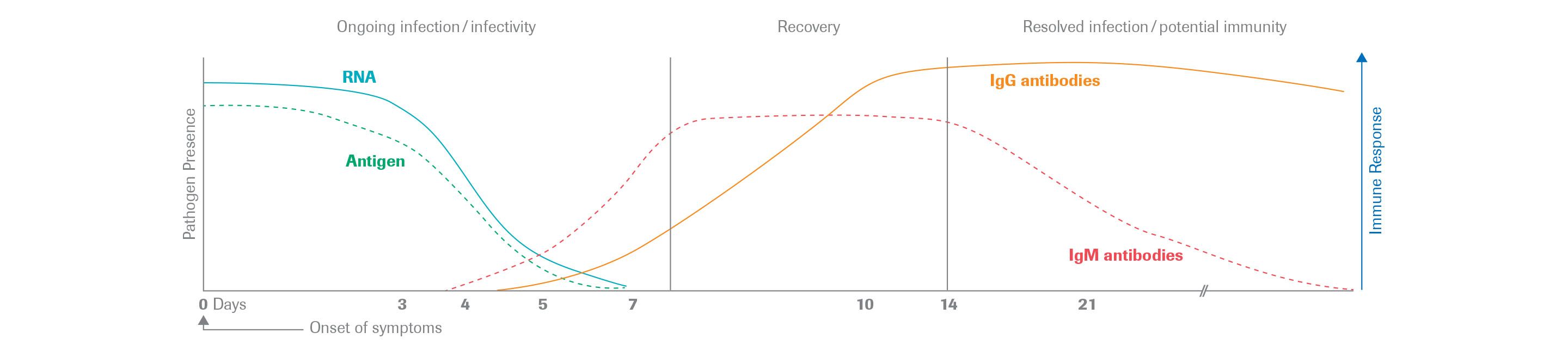
Here is an image of the IgM/IgG profile and the time of serconversion
You are reading a single comment by @deleted and its replies.
Click here to read the full conversation.
 Chalfie
Chalfie
So, talking about the antibody tests and how much the rates vary... how reliable are those antibody tests? Wasn't there a story at some point that the UK got a whole bunch which turned out to be completely useless?
I mean I don't assume countries are doing widespread testing based on something that completely doesn't work, but maybe all of these numbers have to be taken with an extra big grain of salt.